Copenhagen, Denmark – January 21, 2025 – Mermaid Medical Group is proud to announce that the advanced TP Pivot Pro™ has received UKCA (UK Conformity Assessed) approval. The UKCA marking ensures compliance with UK regulatory standards which is essential for the availability of the TP Pivot Pro™ for healthcare professionals across the UK market.
The TP Pivot Pro™, developed by CIVCO Medical Solutions, is a disposable needle guide designed to optimize the minimally invasive, freehand transperineal biopsy approach. Its innovative pivoting coaxial needle enables a 20-degree adjustment from the initial path, simplifying access to the entire prostate gland.
Additionally, it is compatible with most ultrasound probes without the need for additional adhesive materials like tape, offering a secure fit that ensures stability throughout the procedure. It also allows for needle height adjustments without removal from the guide or the patient, further improving ease of use and precision.
The TP Pivot Pro™ is set to make its European debut at the beginning of the year with the support of Dr. Hashim U. Ahmed, a renowned expert in the field, who will be the first to perform procedures using the device in Europe. With his extensive expertise in prostate cancer diagnosis, imaging, and biopsies, Dr. Ahmed’s involvement will play a key role in demonstrating the device’s capabilities during the early cases in the region.
“We are very proud and excited to receive the UKCA approval for the TP Pivot Pro™,” says Michael Kronby, Category Manager at Mermaid Medical. “Patient safety is our biggest priority, and receiving this validation is a major milestone in our journey with CIVCO and the TP Pivot Pro™. Our partnership with CIVCO has been ongoing for more than 17 years, and this achievement reflects the dedication of everyone involved. We are also thrilled to have Dr. Hashim Ahmed support the initial use of the TP Pivot Pro™ in Europe, further underscoring our shared commitment to delivering solutions that live up to the high safety and performance standards in the UK.”
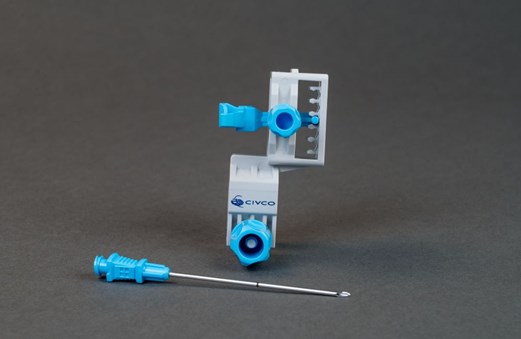
TP Pivot Pro™ by CIVCO Medical Solutions
"CIVCO is excited for the expansion of the TP Pivot Pro™ prostate biopsy guide into the UK market after proven success in the United States, Canada, Australia, and other countries," says CIVCO President, Robin Therme. "TP Pivot Pro™ was designed with and for clinicians to provide a smarter approach to transperineal prostate biopsies. Mermaid Medical has been a trusted partner and distributor of CIVCO products for many years, and we look forward to this next chapter: helping to make prostate biopsies safer with TP Pivot Pro™."
By enhancing the transperineal approach, which has demonstrated a reduction in risks compared to the transrectal method, the TP Pivot Pro marks a significant advancement in the field of interventional urology.
You can learn more about TP Pivot Pro™ here, or reach out to our Customer Service team.
Media Contact:
Anne Sandgaard
Mermaid Medical Group
+45 2194 8508
asa@mermaidmedical.com






