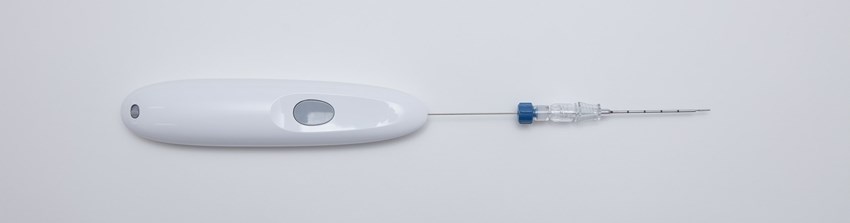
The Single Pass biopsy closure device is a state-of-the-art medical device designed to enhance patient care and improve procedural outcomes. With its advanced technology and user-friendly design, the device offers a safe and efficient method for closing biopsy sites, reducing the risk of complications, and promoting faster patient recovery.
Under this exclusive distribution agreement, Mermaid Medical Group will serve as the sole distributor of Single Pass's biopsy closure device in the United States. Mermaid Medical Group's extensive network and expertise in the medical technology industry make them an ideal partner for Single Pass, ensuring widespread availability and efficient delivery of this innovative device to healthcare facilities across the country.
"We are thrilled to partner with Mermaid Medical Group for the exclusive distribution of our biopsy closure device in the United States," said Bill Colone, CEO at Single Pass. "Mermaid Medical Group's reputation for excellence and their commitment to advancing patient care align perfectly with our mission. Together, we aim to revolutionize biopsy closure procedures and improve patient outcomes."
"We are excited to collaborate with Single Pass and introduce their groundbreaking biopsy closure device to the US market," said Marcus Nielsen, General Manager at Mermaid Medical Group. "This partnership reflects our dedication to providing healthcare professionals with innovative solutions that enhance patient care. We look forward to working closely with Single Pass to make this device widely accessible to medical facilities across the country."
The exclusive distribution agreement between Single Pass and Mermaid Medical Group marks a significant milestone in the advancement of biopsy closure technology. By combining Single Pass's cutting-edge device with Mermaid Medical Group's extensive distribution capabilities, healthcare professionals in the United States will have access to a superior solution for biopsy closure procedures.
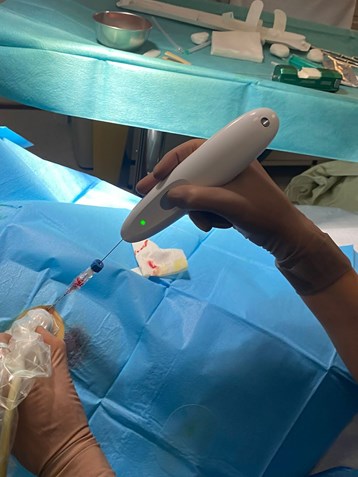
Single Pass would like also to take the opportunity to thank Arne Madsen and Scan MedPartners, who have played a critical role in connecting Single Pass and Mermaid Medical Group facilitating this important partnership that will transform, the biopsy market benefiting patients and payers throughout the US.
Scan MedPartners, an Orange County, CA based Go-To-Market Consulting firm, provides commercial acceleration services via qualified US partner companies, willing to invest in the commercialization of clinically proven disruptive MedTech & Life Science start-ups primarily based out of Scandinavia.
For more information about Single Pass and their biopsy closure device, please visit https://singlepass.co/. To learn more about Mermaid Medical Group and their comprehensive range of medical technology solutions, please visit https://www.mermaidmedical.com/.
About Single Pass:
Single Pass is a leading provider of innovative medical devices, dedicated to improving patient care and procedural outcomes. With a focus on developing advanced solutions for biopsy closure procedures, Single Pass aims to revolutionize the field of interventional medicine.
About Mermaid Medical Group:
Mermaid Medical Group is a privately owned global medical technology company committed to delivering high-quality, innovative solutions to healthcare professionals worldwide. They develop, manufacture, and distribute medical devices to hospitals and end users across Europe, the U.S., and Asia. With a diverse portfolio of medical devices and a strong presence in the industry, Mermaid Medical Group is dedicated to advancing patient care and improving clinical outcomes.
Media Contact:
Marcus Kildegaard Nielsen
Mermaid Medical Group
(720) 440-0008
mkn@mermaidmedical.com
SOURCE Single Pass, Inc.






