Copenhagen, February 13, 2025
Mermaid Medical Group® is pleased to announce the UK launch of the life-saving device from Front Line Medical Technologies Inc, COBRA-OS®.
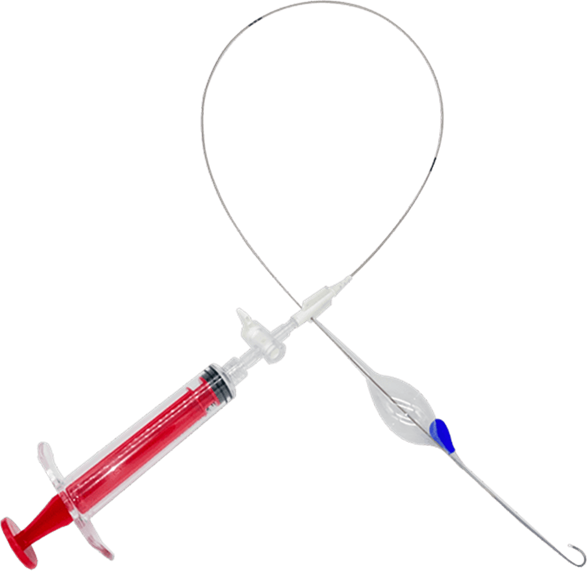
The COBRA-OS® (Control of Bleeding, Resuscitation, Arterial Occlusion System) is an aortic occlusion device used for REBOA and partial REBOA procedures. With its thin 4 Fr size, the COBRA-OS® is currently the smallest device on the market, allowing for smaller arterial access and several other benefits.
Expanding into the UK market has been an important goal for both Front Line Medical Technologies Inc. and Mermaid Medical.
"The United Kingdom represents a crucial milestone in our mission to advance aortic occlusion technology and improve trauma care outcomes," said Dr. Asha Parekh, CEO at Front Line Medical Technologies. "We designed this device to address critical time constraints in emergency settings, where rapid deployment and controlled occlusion can make a vital difference."
Mermaid Medical is proud to partner with Front Line Medical Technologies to make this life-saving technology available to healthcare providers in the UK.
"We are thrilled to strengthen our presence in the endovascular field and, together with Front Line Medical Technologies, reaffirm our shared commitment to improving patient outcomes," said Sebastian Jørgensen, Marketing Director at Mermaid Medical Group. "By offering an expanded portfolio of advanced solutions to our valued partners in the United Kingdom, we mark an important milestone in our mission to support better care."
Front Line Medical Technologies Inc. is a medical device company focused on lowering the barriers to bleeding control and resuscitation worldwide. Committed to advancing emergency medicine and improving patient outcomes in critical situations, the company aims to provide healthcare professionals with innovative solutions. Their mission is to enhance survival rates for critically injured patients, with a vision of becoming a leader in patient survival stories.
Mermaid Medical Group is a privately owned global medical technology company dedicated to delivering high-quality, innovative solutions to healthcare professionals worldwide. The company develop, manufacture, and distribute a diverse portfolio of medical devices, primarily focusing on solutions for diseases in the vascular system and devices used in interventional radiology. With a reach that spans Europe, the U.S., and Asia, Mermaid Medical Group is committed to advancing patient care and improving clinical outcomes.
Media Contact:
Anne Sandgaard
Mermaid Medical Group
+45 2194 8508
asa@mermaidmedical.com





